- Effective Tumor Shrinkage Observed
- Strong Immune Response Activation
- Better Safety and Tolerability
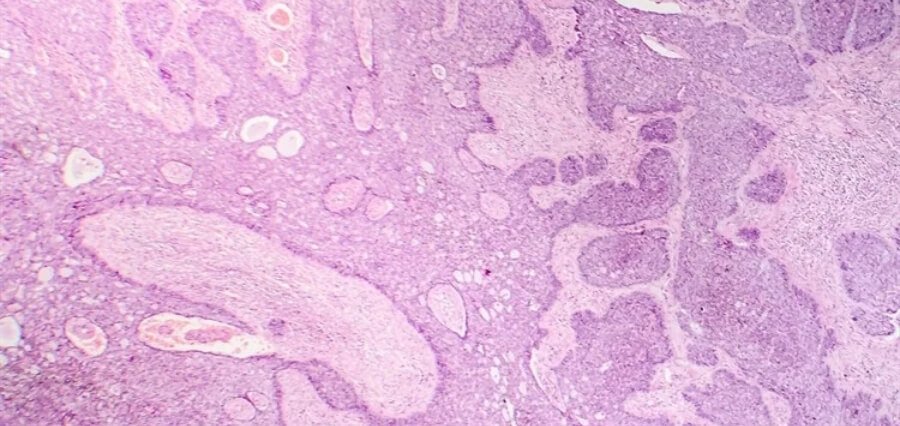
This Phase II clinical trial has just completed and shows promise with this anti-cancer virus, T-VEC: Talimogene laherparepvec, in shrinking refractory cutaneous basal cell carcinomas, BCCs. It is designed to treat locally advanced and hard to resect BCCs. In this process, it appeared that 50% of patients established the evidence that the viruses have made their tumors resectable with an incredible improvement; therefore, was halted early because of its highly exceptional results. T-VEC is genetically modified herpes simplex virus 1, already approved in the U.S. and Europe for the treatment of lesions of unresectable melanomas.
T-VEC was administered to 18 subjects, aged 49 to 92 years, from January 2020 to January 2022, in six cycles of neoadjuvant treatment. The prespecified primary goal was whether this treatment could make the tumors resectable with reconstruction avoided. In nine subjects, the ORR was achieved at 55.6%. There were six CR, four PR, and eight SD among them. Not a single patient demonstrated growth in the size of the tumor upon therapy, and mean in the area reduced by 45.4% at the time of surgery.
Among the two patients whose initial endpoints were positive and negative, respectively, the former had a median reduction of 62.5% in tumors, whereas the latter had a median reduction of 37.7%. The six-month relapse-free and overall measures were 100%, but at median follow-up time of 11 months, new BCCs appeared in two patients.
The four patients who tolerated this T-VEC therapy reported nonserious, treatment-related adverse events. There was a marked, highly significant change in the tumour microenvironment with increases in CD8 + T cells, CD68+ myeloid cells and CD20 + B cells along with a decreased number of CD4 + Treg cells. Such findings promise T-VEC as a modality that activates immunity and, thus, is a promising agent for the augmentation of challenging cases of BCC.